Opioid medications are prescribed to alleviate pain and suffering, but their abuse and misuse is killing more than 33,000 Americans every year. Prescription painkiller abuse is a problem of epidemic proportions and a significant national public health challenge. The good news is, it’s one that states are increasingly taking action against with an important weapon: health information technology.
Electronic prescribing for controlled substances (EPCS) empowers healthcare providers to play a critical role in decreasing opioid fraud and abuse. When its full capabilities are used, EPCS allows providers to see patients’ medication histories at the point of care, which helps them determine if patients are “doctor shopping” or are exhibiting other behaviors associated with drug abuse. EPCS also ensures prescriptions are securely transmitted from provider to pharmacy without the risk of forgery.
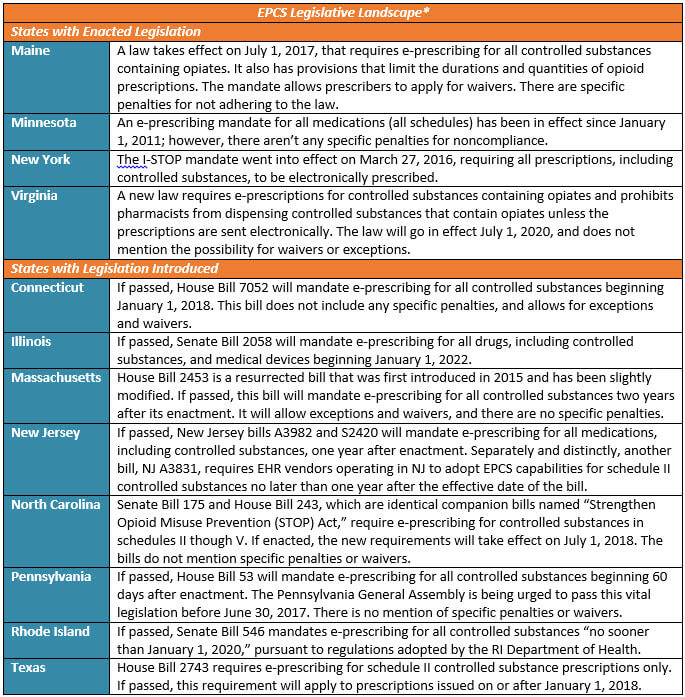
Ultimately, EPCS helps providers proactively minimize opioid drug abuse, which can save lives. That’s why an increasing number of states are following Maine, Minnesota, New York and Virginia in passing legislation that, to varying degrees, mandates the use of EPCS.
The U.S. Drug Enforcement Administration (DEA) approved EPCS in 2010. By 2015, all states had changed their rules to allow EPCS as well. That same year, the number of prescribers using EPCS on the Surescripts network increased by 359%. And in 2016, more than 45 million EPCS transactions crossed our network, which was a 256% increase over 2015. Moreover, prescriber enablement nearly tripled from 5.7% in 2015 to 14.1% in 2016.
In New York State, the I-STOP e-prescribing mandate, which includes an EPCS requirement, took effect in March 2016. Given New York’s increasing enablement levels since the legislation was introduced—73% of the state’s providers are now EPCS-enabled, compared to the national average of 15%—other states can look to New York as proof of how successful EPCS-related legislation can be in driving adoption.
The Virginia Department of Health estimates that 1,000 lives were claimed in the commonwealth during 2016 due to opioid overdoses, an alarming 33% increase over 2015. Virginia’s legislation, which was signed into law on February 23, 2017, requires controlled substances containing opiates to be e-prescribed. It also prohibits pharmacists from dispensing controlled substances containing opiates unless the prescriptions are transmitted electronically.
While EPCS-related mandates have passed in just four states to date, eight others have had similar legislation introduced, including Connecticut, Illinois, Massachusetts, New Jersey, North Carolina, Pennsylvania, Rhode Island, and Texas. See below for detailed information about where each state stands in terms of EPCS legislation.
Looking ahead, we expect to see more states mandate EPCS in some fashion, especially as states like New York show how providers can successfully adopt and use the technology. Given that all of the country’s leading EHRs are now EPCS-enabled, barriers to adoption are lower than ever. Plus, with the myriad of resources available to help guide prescribers through the enablement process step-by-step, saving lives with the help of EPCS has never been easier. In fact, virtually every ambulatory pharmacy in the U.S. is capable of receiving an e-prescription for a controlled substance.
For more information on EPCS and to see where your state ranks in terms of prescriber and pharmacy enablement, click on the maps below. If you are a healthcare provider interested in EPCS, visit GetEPCS.com. To learn how Surescripts, the nation’s leading health information network, is improving patient care and safety with actionable patient intelligence, visit Surescripts.com.
*As of April 6, 2017
View the enablement details for your state:



 Dean Riggott Photography
Surescripts
Dean Riggott Photography
Surescripts
