In 2014, more than 28,000 American lives were cut short by opioid overdose. In fact, opioid overdose quadrupled from 2000 to 2014, and is now the nation’s number-one cause of preventable deathi. But these potent prescription drugs—which include painkillers like morphine and oxycodone—can help to effectively manage chronic pain for millions of people when properly prescribed, administered and monitored.
Naturally, this puts prescribers in a uniquely challenging position. They must responsibly treat truly in-need patients with opioids, while taking steps to prevent abuse or to stop others from accessing the drugs. As Dr. James Holly, CEO of Southeast Texas Medical Associates (SETMA), said, “In Texas, prescribers are questioned when they prescribe controlled substances. They’re also questioned when they don’t. The regulatory burden falls squarely upon the prescriber, and that can adversely affect patients. So, in some cases, clinicians are less willing to prescribe controlled substances.”
The nation’s prescription drug abuse epidemic rages on, but as Surescripts’ 2015 National Progress Report highlights, Electronic Prescribing of Controlled Substances (EPCS) is emerging as a powerful and efficient tool for fighting it. By digitizing the process, prescribers have a valuable tool to prevent fraud and abuse (i.e., drug diversion, “doctor shopping,” etc.), auto-document their work for use during regulatory audits, and help ensure that the right patient gets the right medication without delay.
In 2015, EPCS became legal in all 50 states and Washington, D.C. Since then, its use has increased dramatically. The number of prescribers enabled to use EPCS increased 359% in 2015, resulting in a more than 600% increase in e-prescription volume (or 12.8 million transactions) for these critical medications. New York helped drive nationwide adoption with its I-STOP mandate, requiring that all prescriptions for controlled substances be sent electronically, with a few exceptions.
However, despite this exponential increase in EPCS adoption and its nationwide legality and availability, enablement among prescribers is still low when compared to pharmacies, with just under 6% of prescribers enabled for EPCS in 2015 as compared to 80% of pharmacies.
For prescribers using EPCS, it’s a game-changer. “With EPCS, every single step of the decision-making process as it relates to prescribing a controlled substance is documented automatically. In other words, now, we document it as we prescribe it. When a prescriber is audited, EPCS helps produce the required documentation in an instant, rather than having to manually track everything, which is virtually impossible due to the time and energy involved,” Dr. Holly said.
Texas, where Dr. Holly practices, is currently ranked seventh in the country in prescriber and pharmacy enablement, as well EPCS transaction volume. Click here for more information on state-level EPCS enablement data, including the complete ranking for each state. Below, you can see what types of providers are using EPCS the most. Family practitioners transmit about one-third (32%) of all e-prescriptions for controlled substances.
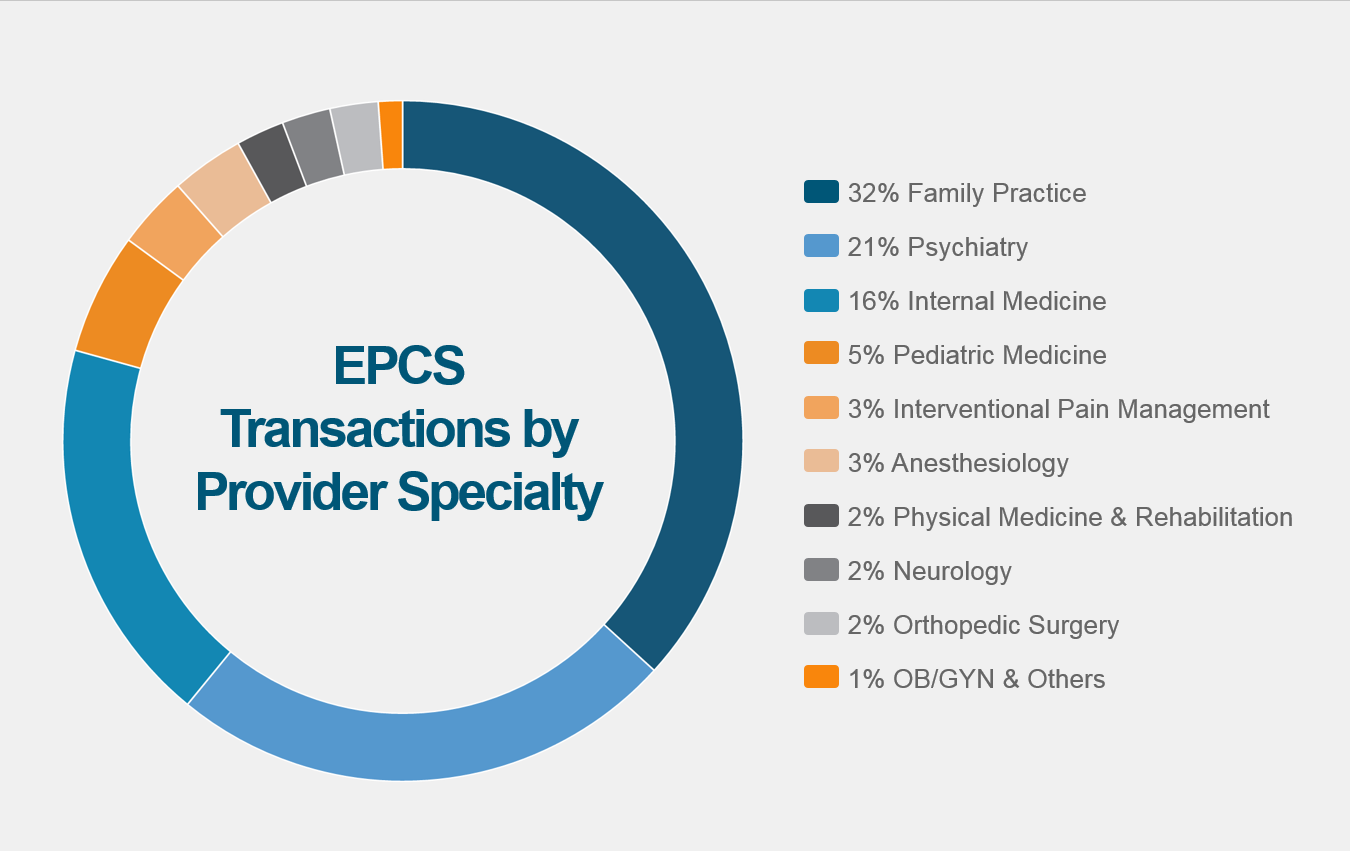
Using the power of Surescripts’ nationwide healthcare information network, prescribers and pharmacies now have access to the electronic tools they need to help fight fraud and abuse of controlled substances while effectively serving the patients who need them. As Surescripts’ 2015 National Progress Report shows, while there’s still a long way to go to 100% EPCS adoption, the growth in enablement over the past year alone indicates a big step in the right direction.
1.i CDC/NCHS, National Vital Statistics System, Mortality: https://www.cdc.gov/drugoverdose/data/statedeaths.html


 Dean Riggott Photography
Surescripts
Dean Riggott Photography
Surescripts
